In the modern pharmaceutical packaging industry, the packaging of capsules and tablets must not only provide excellent sealing and moisture resistance, but also ensure the safety and convenience of the medication. Thanks to its outstanding barrier properties, excellent processing performance, aesthetic appearance, and environmental compliance, 8011 aluminum foil has become the preferred material for pharmaceutical blister pack (PTP) lidding applications.
Role of 8011 Aluminum Foil in Blister Packaging
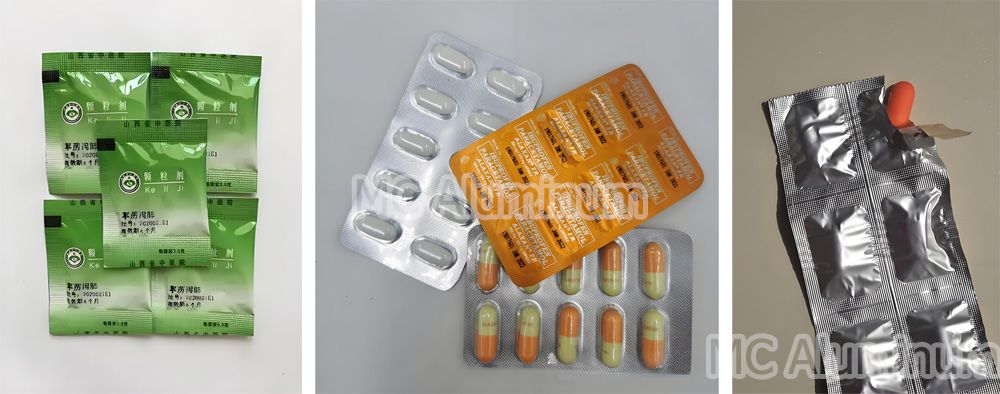
Pharmaceutical blister packaging typically consists of a PVC/PVDC/PP thermoformed base film and a PTP aluminum lidding foil, which is mainly made of 8011 H18 aluminum foil. Capsules or tablets are placed in the cavities of the base film and then sealed with the aluminum foil. When in use, consumers simply press the aluminum foil to pop the tablet out of the cavity, ensuring hygiene and convenience.
Blister packaging for dietary supplements and pharmaceutical tablets/capsules is becoming increasingly common. It is estimated that 60%-70% of future tablet and capsule packaging will adopt blister formats, making it one of the most promising packaging solutions in the pharmaceutical industry.
The core functions of 8011 aluminum foil as a lidding material include:
Blocking moisture, oxygen, and light, preventing pharmaceutical products from deteriorating due to moisture;
Providing excellent sealing properties, ensuring pharmaceutical hygiene and shelf life;
Providing excellent printing, coating, and heat-sealing properties, facilitating brand and batch identification;
Easy to tear and remove, making it convenient for end consumers.

Specifications of 8011 Blister Foil
| Alloy | 8011 |
| Temper | H18, O |
| Thickness | 0.018-0.5 mm |
| Width | 100-1650 mm |
| Surface Finish | One-side bright / double bright / coated / printed |
| Flatness & Cleanliness | Smooth, clean surface with no oil stains, black spots, scratches, or bright lines |
| Pinholes | ≤ 0.1 pcs/m² (for high barrier applications); zero pinholes required |
| Applications | Suitable for tablets, capsules, pills blister packs with PVC rigid film; also used for confectionery, chocolate, chewing gum, and other blister-style packaging |
| MOQ | 1–3 tons |
Chemical Composition of 8011 Aluminum Foil
| Alloy | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Other:Each | Other:Total | Al |
| 8011 | 0.5-0.9 | 0.1-1.0 | 0.1 | 0.2 | 0.05 | 0.05 | 0.1 | 0.08 | 0.05 | 0.15 | remainder |
Excellent Properties of 8011 Pharmaceutical Foil
1. Outstanding barrier performance: Extremely high resistance to water vapor, oxygen, and UV light-critical for maintaining drug stability.
2. Excellent chemical stability: Does not react with most pharmaceutical ingredients; meets pharmaceutical safety standards.
3. Good formability: Supports printing, coating, and heat-seal layer treatment; compatible with high-speed blister packing lines.
4. Environmentally friendly: Non-toxic, odorless, and 100% recyclable, meeting sustainable packaging trends.
5. Stable heat-sealing performance: After coating with a heat-seal layer, it bonds firmly to PVC/PVDC base films without delamination.
6. Puncture resistance: Provides sufficient toughness to protect against punctures from capsules or irregularly shaped tablets during storage and transportation.
7. Good mechanical strength: High tensile and shear strength; suitable for high-speed automated blister packaging in various environments.
Application Fields
Capsule and tablet PTP blister packs
Oral solid dosage packaging
Moisture-proof packaging for medical devices and health supplements
Food, tea, and small-dose powder blister sealing (extended applications)
Quality Control Key Points for 8011 Aluminum Foil in Pharmaceutical Packaging
To meet pharmaceutical-grade packaging requirements, strict control must be applied during production, focusing on the following indicators:
1. Pinhole rate: A critical factor for barrier performance. For aluminum foil of 25 μm thickness, pinholes must be minimized to ensure airtight protection.
2. Surface wettability: Affects the adhesion of printing inks and heat-seal layers. The surface must be clean and oil-free.
3. Mechanical properties: Including tensile strength and elongation, to balance strength and press-through performance.
4. Hygiene standards: Must comply with pharmaceutical packaging regulations-non-toxic, odorless, and chemically inert with the medicine.
