Suppository packaging refers to the process of encapsulating suppository drugs with solid preparations made of suitable matrices. The packaging materials should meet certain requirements to ensure the quality and stability of suppositories. Common suppository packaging materials include aluminum foil, plastic blisters, etc. MC Aluminum can provide 8011 aluminum foil for suppository packaging.
Pharmaceutical grade suppository aluminum foil is specially used for blister packaging of suppository drugs, and its alloy grade is mainly 8011. This kind of aluminum foil has the characteristics of clean, flat surface and neat cutting edges. There are no dense, continuous and periodic pinholes, which ensures the integrity of the packaging. At the same time, 8011 aluminum foil has high heat sealing strength, good sealing, and excellent barrier properties. It is particularly suitable for packaging moisture-sensitive drugs or drugs that need to be sold in hot and humid areas.
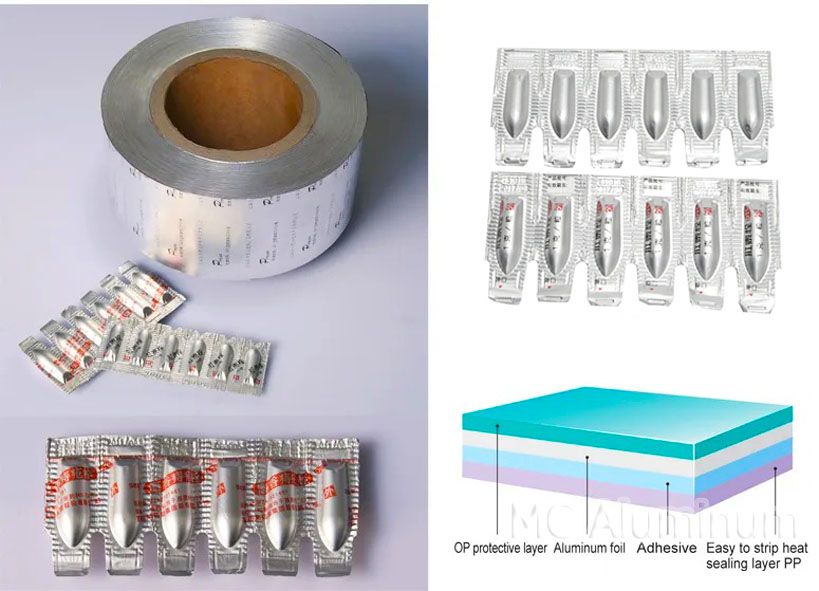
8011 pharmaceutical aluminum foil specifications:
| Model | composite film for suppository packaging |
| Alloy | 8011 |
| Temper | O |
| Thickness | 0.01-0.03mm |
| Width | 10-1600mm |
| Length | 100-1700mm |
| Volume | 1.5-2.5ml |
| Inner diameter | 76mm/152mm |
| Material | AL/PE or PET/PE/AL/PE |
Main advantages of aluminum foil for suppository packaging:
1. High barrier property: It can effectively block air, moisture and light to ensure stable drug efficacy.
2. Good heat sealing performance: It is compounded with PE, PVC and PVDC, with strong sealing to prevent drug contamination.
3. Strong corrosion resistance: It is suitable for the strict storage environment of the pharmaceutical industry to prevent chemical reactions.
4. Comply with international standards: It has passed FDA, EU GMP, ISO 15378 and other certifications to ensure safety.
5. Environmentally friendly and recyclable: It meets the global trend of green packaging and reduces carbon footprint.
6. Good formability: Aluminum foil can be processed by stamping, forming and heat sealing, and is suitable for suppository packaging of various specifications.
Quality inspection requirements for medical aluminum foil for suppository packaging:
1. Material composition:
The chemical composition of aluminum foil must meet relevant standards to ensure its purity and safety.
2. Surface quality:
The surface of aluminum foil should be flat, smooth, free of oil, impurities, pinholes, scratches and other defects.
3. Thickness and size:
The thickness and size of aluminum foil should meet the specification requirements to ensure the sealing and strength of the packaging.
4. Microbiological indicators:
The microbiological indicators of aluminum foil must meet the hygiene standards for pharmaceutical packaging to ensure its sterility.
5. Heavy metal content:
The heavy metal content of aluminum foil must meet the requirements for pharmaceutical packaging to prevent contamination of drugs.
At present, the company's products have been exported to more than 40 countries around the world, including South Korea, India, Denmark, Russia, Bangladesh, Ethiopia, the United States, Canada, Australia, Spain, etc., and the products are deeply trusted and loved by customers.
